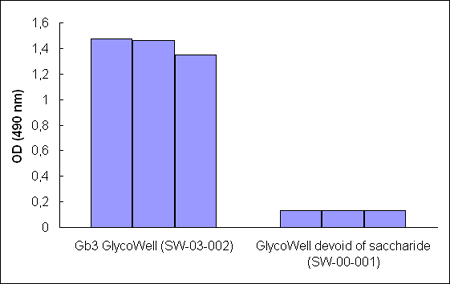
 Figure 1. A Bandeiraea
simplicifolia agglutinin (bs–I)lectin–horseradish peroxidase conjugate binds
GlycoWell™ plates (SW-03-002), but not to a GlycoWell plate devoid of saccharide
(SW-00-001).
Figure 1. A Bandeiraea
simplicifolia agglutinin (bs–I)lectin–horseradish peroxidase conjugate binds
GlycoWell™ plates (SW-03-002), but not to a GlycoWell plate devoid of saccharide
(SW-00-001). |
TechNotes No. 4-1999
Binding of a Bandeiraea simplicifolia agglutinin
— horseradish peroxidase conjugate to b–Globotrioside
GlycoWell™ plates.
Introduction
Binding
of lectins to immobilized saccharides and subsequent inhibition of such a binding
event is often used for evaluating lectin inhibitors. The use of GlycoWell™ plates
facilitates an ELISA assay involving the binding of lectins to the wells. This
TechNote describes the binding of a a–D–galactopyranoside
recognizing lectin, Bandeiraea simplicifolia agglutinin (bs–I)1,
to b–globotrioside (Gb3) GlycoWell™ plates.
Experimental
Results
The
results demonstrate that a b–globotrioside GlycoWell™
plate efficiently binds a a–D–galactopyranoside–recognizing plant lectin,
Bandeiraea simplicifolia agglutinin (bs–I) (Figure 1). Thus, GlycoWell™
plates are suitable for detecting carbohydrate binding properties of proteins.
Materials
 Figure 1. A Bandeiraea
simplicifolia agglutinin (bs–I)lectin–horseradish peroxidase conjugate binds
GlycoWell™ plates (SW-03-002), but not to a GlycoWell plate devoid of saccharide
(SW-00-001).
Figure 1. A Bandeiraea
simplicifolia agglutinin (bs–I)lectin–horseradish peroxidase conjugate binds
GlycoWell™ plates (SW-03-002), but not to a GlycoWell plate devoid of saccharide
(SW-00-001). |